Posted April 29, 2019 in Breast Implants, Implant Rupture
Following FDA restrictions on silicone gel implants in 1992, saline implants were the only available devices for breast augmentation in the U.S. until the approval of silicone gel in late 2006. Unfortunately, most breast implants do not last as long as the women who possess them. If you have undergone or are considering breast augmentation, it is important to note that at some point in your lifetime, you will probably experience implant failure (deflation of saline implants or rupture of silicone implants), requiring surgery for implant replacement.
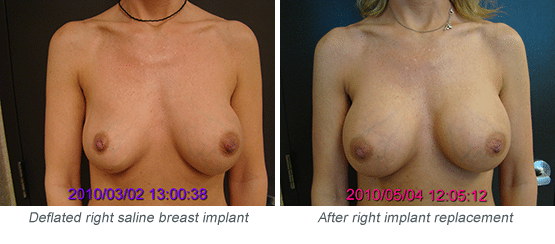
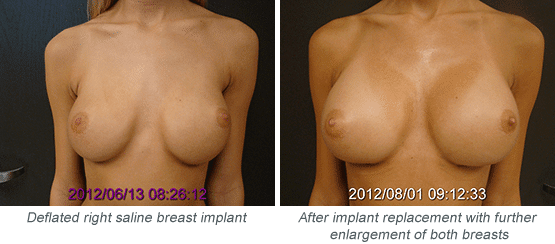
How Will I Know if My Saline Implant Has Deflated?
Because saline implants are filled with saltwater, any leakage will be absorbed by your body. Complete deflation may take a day or two (if there is a large hole in the implant shell) or could take weeks or months (with a smaller leak). Signs of deflation include reduced breast size, reduced firmness of the breast (which may feel soft or “squishy”), and reduced fullness of the upper breast. These findings are generally gradual and progressive. So if you’re not sure whether you have a deflation, look again in a few days or a week. If your breast looks and feels the same, your implant is probably intact. But if it continues to lose fullness and firmness, you should definitely see a plastic surgeon.
Is a Leaking Saline Implant Dangerous?
While there remains some controversy regarding the safety of ruptured silicone implants, we can definitively state that saline implant failure is not dangerous. Saline implants are filled with the same saltwater solution used in IV fluid. In fact, the implants are manufactured as empty shells, and we actually fill the implants with sterile fluid during surgery. If a saline implant deflates, you will be better hydrated—and that’s about it.
Who Pays for Implant Replacement Surgery?
Both FDA-approved saline implant manufacturers, Allergan and Mentor®, provide 10-year warranties on their devices. Unfortunately, these “standard” warranties only cover a portion of your surgical fees; the rest will come out of your pocket.
Unlike most plastic surgery practices, we purchase a Confidence Plus® Platinum Warranty for all of our primary breast augmentation patients; the cost of this is included in your surgical fee. This warranty will cover our entire fee (surgeon, physician anesthesiologist, nationally accredited surgery center on the campus of Texas Health Presbyterian Hospital of Plano, a new saline implant, and postoperative visits) for any deflation that occurs within 10 years of your initial surgery. If you experience a deflation after 10 years, the manufacturer will still provide a replacement implant for free, but they will not pay the other surgical costs.
How Soon Do I Have to Have My Implants Replaced?
While a deflation is not an emergency, it is important to have implant replacement surgery done as soon as possible, ideally within a couple of weeks of the deflation. Capsular contracture, or tightening of scar tissue around the implants, is a risk of an untreated deflation. Since “nature abhors a vacuum,” the capsule of scar tissue, which normally forms around the implants, may tighten following a deflation, requiring a more difficult surgery than implant replacement alone.
Is Implant Replacement Surgery As Difficult As the Original Breast Augmentation?
If there is simply an implant deflation, the replacement surgery is generally straightforward and involves relatively little pain. If there is capsular contracture requiring release (capsulotomy), then the surgery may be more involved.
If I Have to Replace a Deflated Implant, Can I Get Bigger Implants at the Same Time?
Many women choose to have larger implants placed while they are undergoing removal and replacement of a deflated implant. If your implant is under warranty, the manufacturer will cover the costs for one breast surgery, and you will cover the other.
Silicone Gel Breast Implants: Are They Really Safe?
Silicone gel breast implants feel more natural and ripple less than saline implants. But are they safe?
Silicone gel implants were introduced in 1962 and were very popular in the U.S. for three decades. However, in 1992, the FDA (U.S. Food & Drug Administration) placed restrictions on the use of silicone gel following multiple reports of autoimmune diseases (such as arthritis, fibromyalgia, and lupus) reportedly linked to silicone gel. As a result, when Dr. Friedman did his plastic surgery residency at Parkland Hospital in Dallas from 1992 to 1994, they removed many more implants than they placed. Of course, there were also $3 billion in lawsuits against the silicone implant manufacturers at the time.
For the next 14 years, silicone gel became the most thoroughly studied substance on earth. Despite multiple attempts to induce a reaction to silicone gel in lab animals, no inflammatory response could be incited. Human studies failed to demonstrate silicone antibodies. The American College of Rheumatology (rheumatologists treat autoimmune diseases) subsequently concluded that there is no demonstrable link between silicone breast implants and any autoimmune disease process.
In November 2006, the FDA reversed its 1992 decision and approved Allergan (formerly Inamed) and Mentor® silicone gel implants for use in the United States. However, the FDA has attached some strings to the use of silicone gel. While there are no age restrictions for saline implants, silicone breast implants are only allowed in women aged 22 and older. In addition, the FDA has stated that while intact, silicone gel implants are safe, however, the safety of ruptured silicone gel implants has not been conclusively established. Therefore, the FDA recommends MRI (magnetic resonance imaging) scans of the breasts at three years postoperatively and every two years after that. These exams are expensive (at least $1500) and are not covered by insurance. As a result, most women are choosing not to undergo regular MRIs postoperatively.
Given the fact that the manufacturers offer a 10-year implant warranty that covers most or all surgical costs if the silicone gel implants rupture, an MRI prior to your 10-year implant anniversary is probably a good idea. It is better to have the manufacturer pay the cost of replacing a ruptured implant than to pay it on your own.
So is silicone gel safe? If the implants are intact, the FDA says, “Yes.” If they’re ruptured, the FDA says, “We’re not really sure.” Dr. Friedman does not think that silicone gel is an evil substance. As long as you understand that there is still some residual controversy surrounding silicone gel, he thinks it’s very reasonable to use. However, if silicone implants still make you nervous, then saline breast implants remain an excellent alternative for most women.
Is MRI Screening Really Necessary?
The FDA currently recommends MRI (magnetic resonance imaging) evaluation of the breasts three years after breast augmentation and every other year following this (i.e., at three, five, seven, nine, etc. years after surgery). Given the expense and inconvenience of routine MRIs, only 10 percent of women with silicone implants currently follow the FDA’s screening guidelines. Most simply look down every other year and say, “Yep. They still look good.”
Intraoperative photos of ruptured silicone gel implants
Ruptured Silicone Gel Implant Manufactured Before 1992
![]()
Ruptured Silicone Gel Implant Manufactured After 2006
![]()
The real question is: how often do the screening MRIs actually detect a rupture? Researchers at Memorial Sloan-Kettering Cancer Center in New York published their data in the Journal of Plastic and Reconstructive Surgery. The researchers performed screening MRIs on 383 reconstructive breast surgery patients. They demonstrated a total of zero silicone implant ruptures in 631 implants at three years postoperatively. They also demonstrated a total of zero ruptures at five years postoperatively. Their conclusion: routine MRI screening of silicone breast implants at three and five years after surgery appears to be unnecessary.
![]()
The above image shows an example of intact (left) and ruptured (right) implants, both removed from the same patient during the removal and replacement of her silicone implants. The rupture was detected during a routine MRI evaluation.
![]()
MRI demonstrates the “linguini sign,” curving black lines that are characteristic of silicone gel breast implant rupture.
Does This Mean That I Will Never Need an MRI?
Not at all. They should be performed for the following three reasons:
- This study strictly included asymptomatic women. Women with pain, implant deformity, new breast lumps, etc. were excluded from this study. If you have silicone gel implants and are experiencing problems with them, MRI is very effective at detecting silicone gel breast implant ruptures.
- This study only offers conclusive data for three-year and five-year screening MRIs. Preliminary results suggest a rupture rate of 2.3 percent at seven years and 15.1 percent at 10 years. Silicone gel implants can and do rupture; they just tend not to rupture in the first five years.
- The FDA-approved manufacturers of silicone gel implants (Allergan, Mentor®, and Sientra®) offer a 10-year warranty on their products. For example, Allergan (whose implants I typically use) will provide a new implant at no cost to you and will pay most or all of the surgical, anesthesia, and facility costs to replace a ruptured silicone gel implant–provided that the rupture is documented within the first 10 years of surgery.
Based on this information, Dr. Friedman recommends the following guidelines for women who have undergone breast augmentation with silicone implants:
- If you have new breast pain, lumps, or an implant deformity, then appropriate evaluation (which may include MRI) is strongly recommended.
If you are NOT experiencing any breast or implant-related problems:
| Projected Rupture Rate | MRI | |
| 3 Years | 0% | Unnecessary |
| 5 Years | 0% | Unnecessary |
| 7 Years | 2.3% | At your discretion |
| 10 Years | 15.1% | YES |
Dr. Friedman strongly recommends that all women with silicone gel breast implants undergo MRI evaluation before the tenth anniversary of their breast surgery. There is a 15.1 percent chance that you will have a ruptured implant (based upon the above study) and a 100 percent chance that the manufacturer will cover most or all of the costs of implant replacement surgery. If you wait more than 10 years and a rupture is detected, the manufacturer will still provide you a new implant. But all other surgical costs will be your problem—not theirs.
If you have more questions about what to do if your breast implants fail, call our office at (469) 467-0100 or fill out our online contact form.